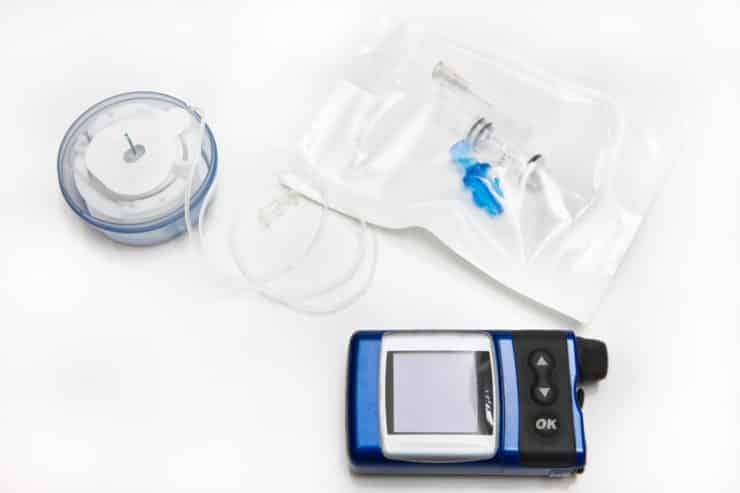
Medtronic MiniMed Insulin Pump Recall Lawsuit
In November 2019, the U.S. Food and Drug Administration (FDA) announced that device maker, Medtronic has issued a Class I recall of their Medtronic MiniMed Insulin Pumps. The recall may affect an estimated 463,000 insulin pumps in the U.S. and was issued due to a potential for incorrect insulin dosing.
Affected insulin pumps may have a missing or broken retainer ring which normally helps to lock the insulin cartridge containing the supply of insulin into place. If the cartridge is not locked properly, the pump may over deliver or under deliver insulin. This may result in a serious medical condition of hypoglycemia or hyperglycemia. Either condition may be serious or life-threatening.
The FDA has stated that they have received 57,000 complaints about the pumps including 2,175 injuries and one death. The Agency has been critical of Medtronic for delaying recall of the pumps and announced expansion of the recall in October 2021.
Medtronic may be facing multiple lawsuits filed by people and loved ones of those who were injured or who died as a result of a MiniMed Insulin Pump malfunction.
Medtronic MiniMed Recall
MiniMed insulin pumps are small drug delivery devices which provide a supply of insulin to diabetics as part of their disease management. On November 21, 2019, the FDA issued a safety alert notifying the public and medical professionals of the MiniMed Insulin Pump recall. The recall was later expanded in October 2022. Both notifications labeled the recalls as Class I. A Class I recall indicates a high potential for serious injury or death.
The recall was based on a potential for inaccurate dosing which may result in overdose or underdose of insulin. This may occur due to a missing or broken retainer ring which has been dislodged or fails to lock the insulin reservoir or cartridge in place. If not locked securely, the device may over or under deliver insulin to the patient. Insulin over dosage may cause hypoglycemia, while under dosage may cause hyperglycemia.
The recall affects an estimated 463,000 MiniMed 600 series insulin pumps in the U.S. including:
- Model 630G (MMT-1715) manufactured before October 2019
- Model 670G (MMT-1780) manufactured before August 2019
The MiniMed model 630G pumps were approved for use in persons 16 years of age or older and 670G pumps were approved for use in patients over the age of 7, indicating that Type 1 diabetics who are children, adult or elderly and who are using MiniMed 600 series pumps may be at risk.
FDA MiniMed Recall Instructions
The November 2019 FDA safety alert notification about recall of certain Medtronic MiniMed pumps included instructions to medical professionals and patients using the pumps.
Users of the affected pumps were advised to:
- Examine the retainer ring of their pump and:
- Stop using the pump if it appears to be damaged, loose, or missing
- Stop using the pump if the reservoir cartridge does not lock into place
- If pump use is discontinued, follow health practitioner instructions for manual insulin dosing
- If pump is dropped, check pump and retainer ring for damage
- Check pump retainer ring and ensure cartridge is properly locked at every set change
- Report signs or symptoms of hypoglycemia or hyperglycemia to a healthcare professional right away
Hypoglycemia may cause dizziness, confusion, weakness, irregular heart rhythm, seizures, loss of consciousness, or death.
Hyperglycemia may cause extreme thirst and fatigue, nausea and vomiting, shortness of breath, weakness, loss of consciousness, coma, or death.
Previous Medtronic Recalls
The November 2019 Medtronic insulin pump recall is only the latest in a history of recalls involving MiniMed insulin pumps.
Past recalls have included:
- In November 2019, the FDA issued a recall for certain MiniMed pumps with remote controllers over concerns about cybersecurity of the controller
- In June 2019, the FDA warned about a possible risk of hacking involving the MiniMed pumps after it was discovered that devices programming may lack cybersecurity protections
- In September 2017, Medtronic issued a recall for the MiniMed Infusion pumps due to cases of hypoglycemia which had involved a blocked membrane
- In September 2015, Medtronic recalled the MiniMed 620G and 640G pumps due to a problem with the drive motor in the pump and a separate recall over a malfunctioning timer unit
- In September 2014, Medtronic recalled the MiniMed Paradigm insulin pump after programming errors resulted in patients receiving the maximum insulin dose
- In June 2013, the company recalled MiniMed Paradigm infusion sets after it was discovered that fluid entering tubing vents could prevent the pump from priming properly. The following month, the Paradigm reservoirs were recalled for a similar problem
- In 2009, Medtronic recalled MiniMed Quickset infusion sets for the Paradigm pump after a manufacturing defect was discovered that could result in incorrect insulin delivery.
The current recall concerns a newer type of pump which are designed to be used as part of a “closed-loop” system which automatically detects blood sugar levels and delivers insulin without the user’s intervention. The automated system was approved in as the MiniMed 670G in September 2016 as the first “artificial pancreas” type pumps. Its use was expanded to users over the age of 7 years in June 2018.
The 630G was first approved in 2016 and included an “automatic suspend” for glucose levels that were too low, but it was not a “fully automatic” system as insulin doses were preset. The 630G was expanded to include a “SmartGuard” system in June 2017 with an additional sensor.
Multiple Medtronic MiniMed Insulin Pump Lawsuits
Even though Medtronic’s technology has continued to advance, serious risks have continued to occur. These risks may pose threat of severe or permanent injury or even death to the users.
Medtronic may be facing multiple lawsuits for injuries caused by its MiniMed Insulin pumps in addition to other lawsuits filed against the company. In September 2022, an investor lawsuit was filed against Medtronic accusing the company of failing to disclose insulin pump problems.
No settlements for Medtronic MiniMed Insulin Pump Lawsuits or other lawsuits against the company have been announced.